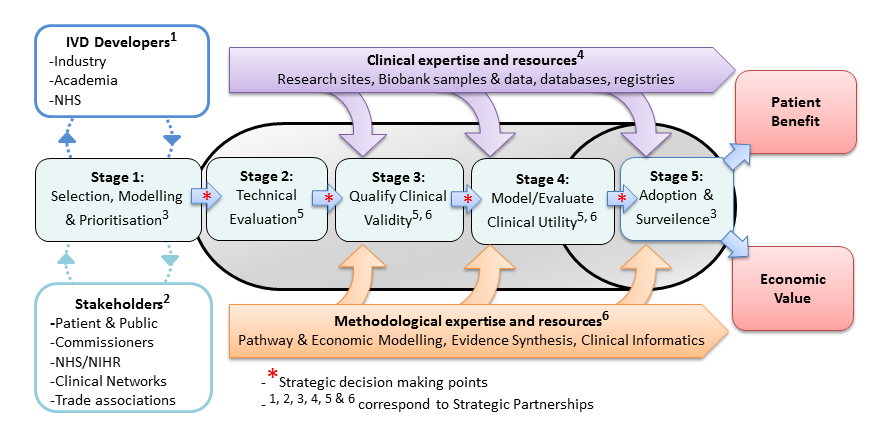
Using decision modelling and value of information analysis (VoI) early in the development of new technologies has proven an effective way of ensuring that innovation leads to high value interventions for NHS patients. There is an acute need to improve efficiency in the development of new genomic and precision medicine technologies.
With this in mind, the UK National Institute for Health Research launched four Diagnostic Evidence Co-operatives (DEC) in 2013 based at the Universities of Leeds, Newcastle, Imperial College London and Oxford. They were founded on the premise of early academic methodological engagement for programme development. A summary of their experiences in the use of early modelling is now published in the Medical Decision Making journal. Following the success of the original four DECs, the NIHR have launched an expanded network of next-generation DECs called the NIHR MedTech and In vitro diagnostic Co-operatives
Building on the earlier methodological work by Karl Claxton of the University of York and others, the Leeds DEC undertook two proof-of-concept studies led by Peter Hall to better understand the ability of early modelling, VoI and Bayesian Decision Analysis to (a) design a clinical trial of a novel diagnostic technology and (b) shape a diagnostic development portfolio for a specific disease area.
(A) The OPTIMAprelim trial used early modelling and VoI to select highest the priority genomic test from a list that included Oncotype DX, Prosigna and Endopredict, for further study in a randomised controlled trial (OPTIMA) of chemotherapy versus test-directed chemotherapy for early breast cancer.
(B) The AKI-Diagnostics project used clinical pathway modelling to understand the priorities for a UK research programme into diagnostic tests for Acute Kidney Injury in Critical Care.
Following the success of the original four DECs, the NIHR have launched an expanded network of next-generation DECs called the NIHR MedTech and In vitro diagnostic Co-operatives