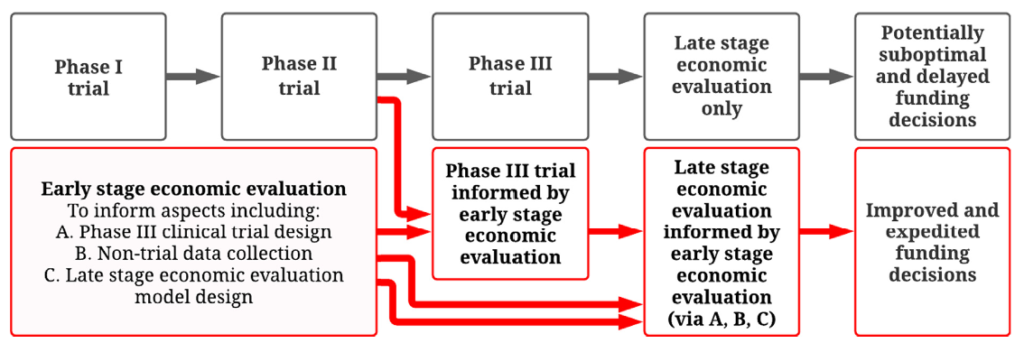
In many countries, economic evaluation is conducted using decision analytic models and must be undertaken in order to assess value for money and to make funding decisions for vaccines and other pharmaceuticals . These analyses are routinely conducted only at a late stage once the vaccine development process is complete or near complete (e.g. after phase III trials). Although economic evaluations are critical to major national funding decisions for vaccines and other drugs, there is currently no systematic planning process to meet their data requirements, which often involve many model inputs of varying uncertainty.
It is not until completion of late stage economic evaluations that we determine if the level of uncertainty in each model input is too large to reliably establish if the vaccine offers value for money. In many cases substantial uncertainty remains when reimbursement decisions are required. At this point, decision makers are faced with a dilemma. They can either recommend the vaccine based on imperfect available data and risk making a suboptimal decision (e.g. over-paying for a vaccine or not funding a vaccine that would be more beneficial than expected) or delay a reimbursement decision until more data are collected. Delays in funding vaccines can result in vaccine-preventable morbidity and mortality, while inefficient allocation of healthcare budgets can lead to less funding for other important healthcare programs.
How can early stage economic evaluation help address this problem?
Originally published December 2021 in Vaccine journal: Newall AT, Beutels P, Tuffaha HW, Hall PS, Jit M. How can early stage economic evaluation help guide research for future vaccines?