
The MABEL Trial
The MABEL trial investigated the effectiveness and cost-effectiveness of low-dose, low-acting oral morphine in patients with chronic breathlessness. Despite chronic breathlessness being a global problem with disabling symptoms, its health economic implications have not previously been comprehensively studied.
143 participants (75 morphine and 67 placebo) were randomised in 11 UK hospitals as part of the MABEL trial.
Health Economic Methods Used
- Quality-adjusted life-years (QALYs) were elicited from the EuroQoL EQ-5D instrument.
- Health resource use (HRU) data were collected via a modified version of the UK Cancer Cost Questionnaire.
- Probabilistic sensitivity analysis & missing data handled together using the bootImpute package in R software, which combines the following tools:
- Non-parametric bootstrapping
- Multiple imputation with chained equations
- Generalised linear model regressions for costs and QALYs (pooled across bootstrapped datasets)
Results
In its base case 56-day results, the health economic analysis found non-significant differences in costs and QALYs between the morphine and placebo arms, equal to £24 (95% CI: −£395 to £552) and 0.002 (95% CI: −0.004 to 0.008) QALYs. Interestingly, however, HRU patterns differed: there were more inpatient and fewer outpatient services used by the morphine versus placebo patients.
Probabilistic results showed a high level of uncertainty surrounding the incremental cost-effectiveness ratio (ICER) of £12,000/QALY and a 54% probability of cost-effectiveness at a £20,000 willingness-to-pay (WTP) threshold.
The Cost-Effectiveness Plane presents bootstrapped results of cost differences vs QALY differences spread across all quadrants of each graph around the [0,0] point (see publication links for more information on scenario analysis ), indicating high uncertainty and no clear effect of morphine on costs or QALYs by Day 56:
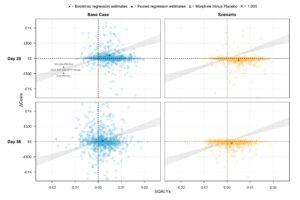
These results are consistent with the trial’s main clinical findings, i.e., no evidence that morphine alleviates breathlessness. The study contributes to palliative care literature by presenting high-quality and comprehensive clinical and economic results that can be used to inform future research and economic modelling of chronic breathlessness.
Publication Links
The Mabel within-trial health economic analysis publication is available here (BMJ Open): https://bmjopen.bmj.com/content/15/11/e102124
Main clinical trial publication (The Lancet): https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(25)00205-X/fulltext
