Lock-down, apatite chemistry and the importance of metastudies
The SARS-Cov-2 pandemic has had a significant impact on a great many areas of life. In the UK, building shutdowns in 2020 meant that many scientists had to rapidly rewrite their research plans for summer. Lab work became challenging, and in many cases, was simply not possible. However, there can also be opportunities with challenges like this. The summer-long building shutdown meant that I ended up spending time working on something which might, normally, have ended up putting on the back-burner. Earlier this week, the results of this ‘homework’ project were published in Lithos.
Apatite is a really interesting mineral. As a phosphate, it is typically a minor phase (there are only small amounts of it) in most rocks. It is found everywhere though, and the fact that it can happily hoover up lots of the more obscure elements of the periodic table means that it can be incredibly useful to Earth Scientists. This diverse chemistry means that apatites can provides insight into processes occurring on the Earth, and elsewhere in our solar system. Some of the oldest minerals on Earth are recycled apatites. These mineral grains tell use about conditions on the early Earth, and may constrain key processes in Earth’s evolution. Apatite records magmatic processes occurring in Earth’s interior today, and may also provide insight into how the Moon formed.
One exciting thing apatite might tell us is oxygen fugacity. Oxygen fugacity, or f(O2) is a hard thing to describe. You can think of f(O2) as a measure of how much oxygen is available to react with other things. This allows us to predict, for example, if multivalent elements like iron exist as Fe2+ or Fe3+. Despite the importance of f(O2) in modelling planetary processes, it remains very difficult to constrain. Some time ago it was proposed that the amount of manganese, Mn, in apatite might be directly related to f(O2). This means that in igneous rocks, apatite could record f(O2) of magmas from which it crystallised, and by extension, provide insight into f(O2) in planetary interiors.
Andy Miles noted that the Mn content of apatites in the zoned Criffel granite appeared to be unrelated to overall Mn content of the rock, or any other obvious parameters. Instead, apatite Mn appeared to vary with f(O2). Examination of published data appeared to support this. Mn content of apatite could be correlated with f(O2), at least in the small subset of rocks where f(O2) had been reasonably well constrained by other methods. I became involved in this work while Andy was a PhD student at Edinburgh. There is a fairly obvious reason why the concentration of Mn in apatite might vary with f(O2). In nature, Mn can exist in many oxidation states, including Mn2+ and Mn3+. The crystal structure of apatite suggests that it should readily incorporate Mn2+. However, it is much harder for apatite to incorporate the substantially smaller Mn3+. So, any change in f(O2) which results in a change in the ratios of Mn2+ and Mn3+ could have a direct effect on the ability of apatite to incorporate Mn as it crystallises.
So, the Mn in apatite oxybarometer was born. Or at least suggested (note the question mark in the paper title). It’s worth investigating, as this would be a simple, elegant way of estimating f(O2). The paper has been cited a lot, which just goes to show how useful any method of estimating f(O2) is. However, things get more complicated when you look at them in more detail. Tom Stokes spent his PhD investigating apatite chemistry, and specifically, researched what apatite can tell us about magmatic f(O2). Worryingly, Tom failed to see any evidence to support a Mn in apatite oxybarometer, as described in a paper in Chemical Geology.
Tom did a series of carefully designed high pressure/temperature experiments, which basically showed (contrary to what would be hoped) that as you change f(O2), partitioning of Mn between a magma and apatite (i.e. how much Mn goes into the apatite structure) does not change. In fact, some rather nice X-ray absorption measurements which Tom conducted at the Diamond light source explain why. The proportion of Mn2+ and Mn3+ in most magmatic systems doesn’t change that much. Simply put, over a range of terrestrial conditions, Mn remains rather dull. The small amounts of it, and it’s insensitivity of f(O2), mean that there is no driving force for a change in apatite chemistry. So, why are variations in the Mn content of apatite noted in places like the Criffell pluton. Well, Tom explained this as well. By performing experiments in different systems he was able to demonstrate that it is not apatite that matters at all. Magma or melt structure is what matters. When the composition of a magma changes, the ‘structure’ can change considerably. Very silica-rich magmas, including some of those at Criffell, are increasingly ‘polymerised’. They consist, in part, of very long chains. As they get more silica-rich, this polymerisation increases, driving Mn out of the melt and into apatite. The very fact that apatite can incorporate lots of elements is the reason Mn content varies. Apatite simply hoovers up what is around when it is growing.
It’s a nice paper. The work highlights (again…this is not the first time this has been suggested by any means) how important melt chemistry is. The work also again highlights how useful apatite is. It might not record changes in f(O2) but it is very sensitive to changes in magma. However, people keep using the Mn in apatite oxybarometer. It’s easy to use, and gives you an estimate of something really important which is really hard to measure.
This is where the new paper comes in. I thought a fun, independent way to assess the Mn in apatite oxybarometer would be a metastudy of published data. Metastudies are common in many areas of science. A bit less so in geochemistry. A metastudy is simply a study of other studies. The idea here was simple. I could spend time away from the lab collecting as much data on apatite as I could find. I trawled the literature, collating as much information as I could find on apatite, and on the igneous rocks in which it was found. Of course, everything is published in a slightly different format, and to be honest, this step was really painful. But, it was lockdown and it was a useful distraction. Then I had to go through each dataset and convert the data into a consistent format. Again, painful, but there was light at the end of the tunnel. And the next step was then playing with an enormous dataset of thousands on individual analyses. Each one the result of someone’s hard work in a lab. Each one from a separate grain of apatite, from a different rock from across the surface of the Earth. All meta-tagged.
And the result. Amazingly, you can see some really interesting trends in the data. Basically, Tom’s work is proven correct. Magma composition and structure (here estimated from whole rock data) has a significant control on apatite chemistry, especially Mn content. Despite all the factors that can affect apatite composition (magma composition, pressure, temperature, exact point at which apatite crystallises, the presence of volatiles, and on and on) a strong control of melt structure is really evident in data set. A metastudy of natural apatite fully supports the lab work, which, as an experimental person, is nice to see. It also highlights how important metastudies are. That is apparent from the news, though, when you are in the middle of a pandemic.
So, what else? Well, a massive dataset allows you to then look at lots of other fun stuff too. I looked at a range of other multivalent elements, including europium and cerium. The figure below shows some of the fun you can have doing this.
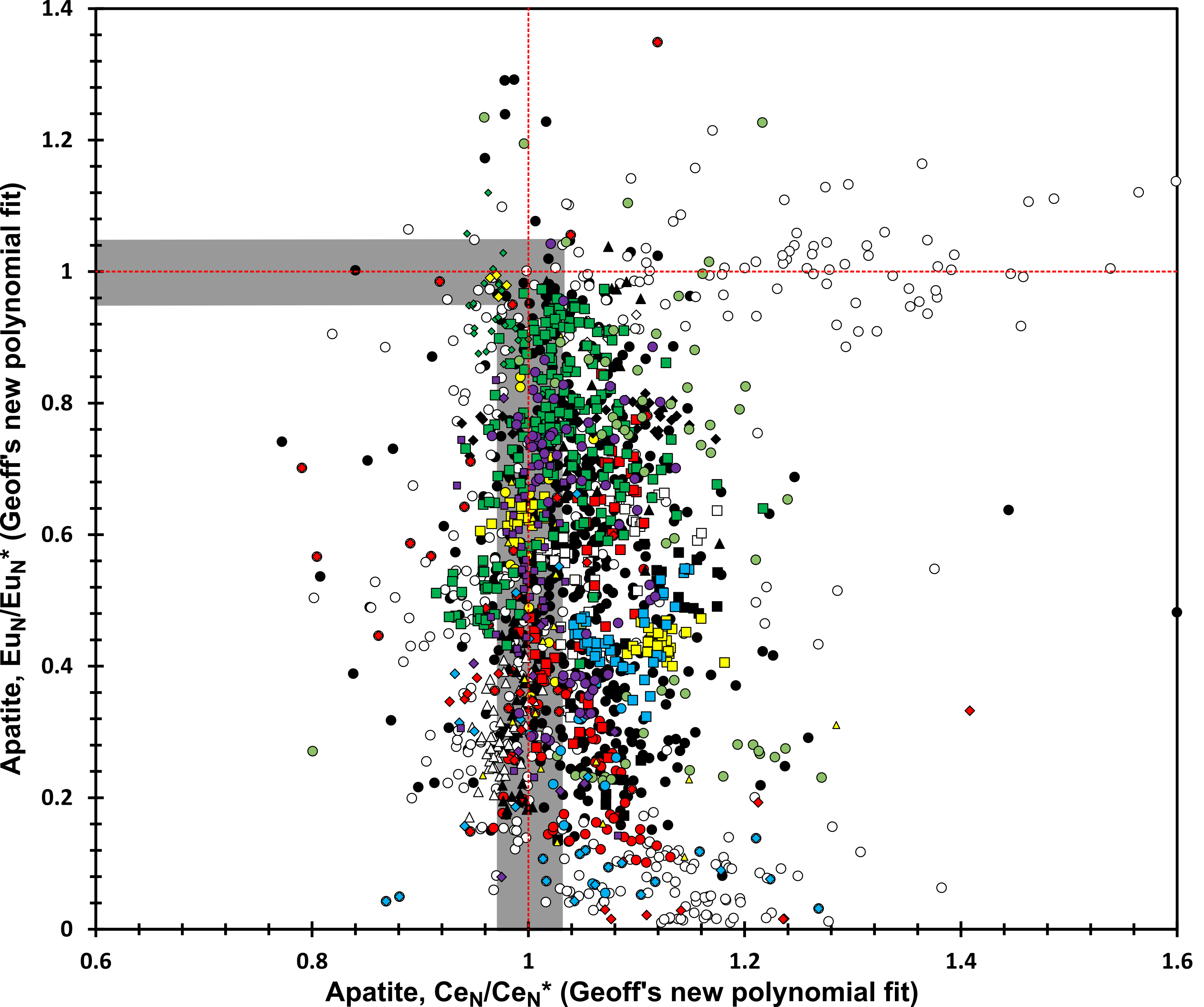
Again, things don’t look good. Apatite does not reliably record f(O2). It does, however, tell you lots about magmas, which we explore in the paper. And the metastudy does demonstrate quite how useful apatite is. Just, sadly, not for f(O2).




Recent comments