EMT: Epithelial-to-mesenchymal transition – Another piece in the puzzle of understanding metastasis formation?
Starting my PhD in the field of developmental biology, I was very excited to attend the seminar at the IGMM by the award-winning guest speaker Cédric Blanpain, a pioneer in lineage tracing and tumour research at the Université Libre de Bruxelles, Belgium. For his outstanding work in the field of stem cell and cancer development he was voted one of the “10 Scientists Who Matter The Most” in 2012 by Nature magazine editors highlighting his revolutionary contributions to the field. His recent studies focus on the mechanisms that regulate tumour transition states, a very important topic that could shed light on the complex nature of cancer development and the steps a cancer cell has to undergo in order to metastasise. Cédric Blanpain illustrated in his talk the multifaceted traits of tumour cells and why it is necessary to study all of them to fully understand tumour progression.
Cancer still represents the second leading cause of death globally, with tumour metastasis being primarily responsible for the high morbidity and mortality rate. Metastasis is the process whereby cancer cells break away from the original tumour and travel through the blood stream to enter and colonise distant parts of the body, eventually developing secondary tumours (Revenco et al., 2019). Metastasis formation is coupled to a range of very complex processes that are still poorly understood. Our knowledge so far is limited to the basic mechanisms and describes a cascade of events where a cell has to undergo various morphological and biochemical changes in order to be able to metastasise: First of all, the cell has to lose its cohesive properties and detach from neighbouring cells. Secondly, the cell has to migrate from the microenvironment of the primary tumour and enter the blood stream in order to circulate to distant tissues and organs. Thirdly, the cell has to exit the blood stream and invade the new environment to establish secondary tumours (Revenco et al., 2019). The lack of understanding regarding metastasis formation, cancer heterogeneity and paucity of early detection methods all remain major challenges in cancer treatment. Therefore, researchers all over the world are dedicated to putting together all aspects of these complex mechanisms and pathways piece by piece to more comprehensively understand the complex nature of cancer development and progression.
Blanpain might have found another piece to the puzzle: a phenomenon known as EMT (Epithelial-to-mesenchymal transition) in which epithelial cells convert into mesenchymal cells. This highly dynamic process allows cells to lose polarity and cell-cell adhesion and gain migratory and invasive properties (Yeung and Yang, 2017). Classical EMT is a crucial programme for cells during embryonic development, allowing stem cells to acquire multipotency and the ability to differentiate into a variety of cell types (Brabletz et al., 2018). However, EMT can be re-activated in cancer and contribute to tumorigenic progression, highlighting EMT as a potential new target in anti-cancer therapies (Yeung and Yang, 2017).
Blanpain and his research group developed genetically engineered mouse models with KRASG12Dexpression and p53 deletion which give rise to tumours that were fluorescently tagged with YFP to assess EMT in two classes of primary skin SCC (squamous cell carcinoma), namely interfollicular skin epidermis (IFE) and hair follicle (HF) (Revenco et al., 2019). These studies revealed that EMT occurs in different stages referred to as hybrid EMT states which are characterised by the co-expression of epithelial and mesenchymal markers accompanied by intermediate morphological and transcriptional features (Pastushenko et al., 2018). In HF-derived SCCs, these intermediate stages were analysed by immunostaining of tumour cells with EpCAM or Keratin14 (K14) as epithelial markers and Vimentin (Vim) as a mesenchymal marker. Loss of EpCAM expression coincided with a gain of Vimentin expression indicating the first switch to a mesenchymal state. Co-expression of both epithelial and mesenchymal markers in different sub-populations represented hybrid states and complete loss of K14 expression corresponded to full EMT tumour cells (see Figure 1) (Pastushenko and Blanpain, 2019). The molecular switch in gene expression from epithelial to mesenchymal is triggered by a complex regulatory network of activating transcription factors (EMT-TFs) mainly involving SNAI1/2, TWIST and ZEB1/2 families, which were found to be upregulated in EMT transition states (Pastushenko et al., 2018). EMT-TFs are important mediators of cellular plasticity, a major hallmark of tumour cells that allows them to rapidly adapt to constantly changing environmental conditions (Gupta et al., 2019). Activation of the aforementioned EMT-TFs also resulted in increased stimulation of cell mobility, thereby promoting tumour invasion and dissemination (Brabletz et al., 2018).
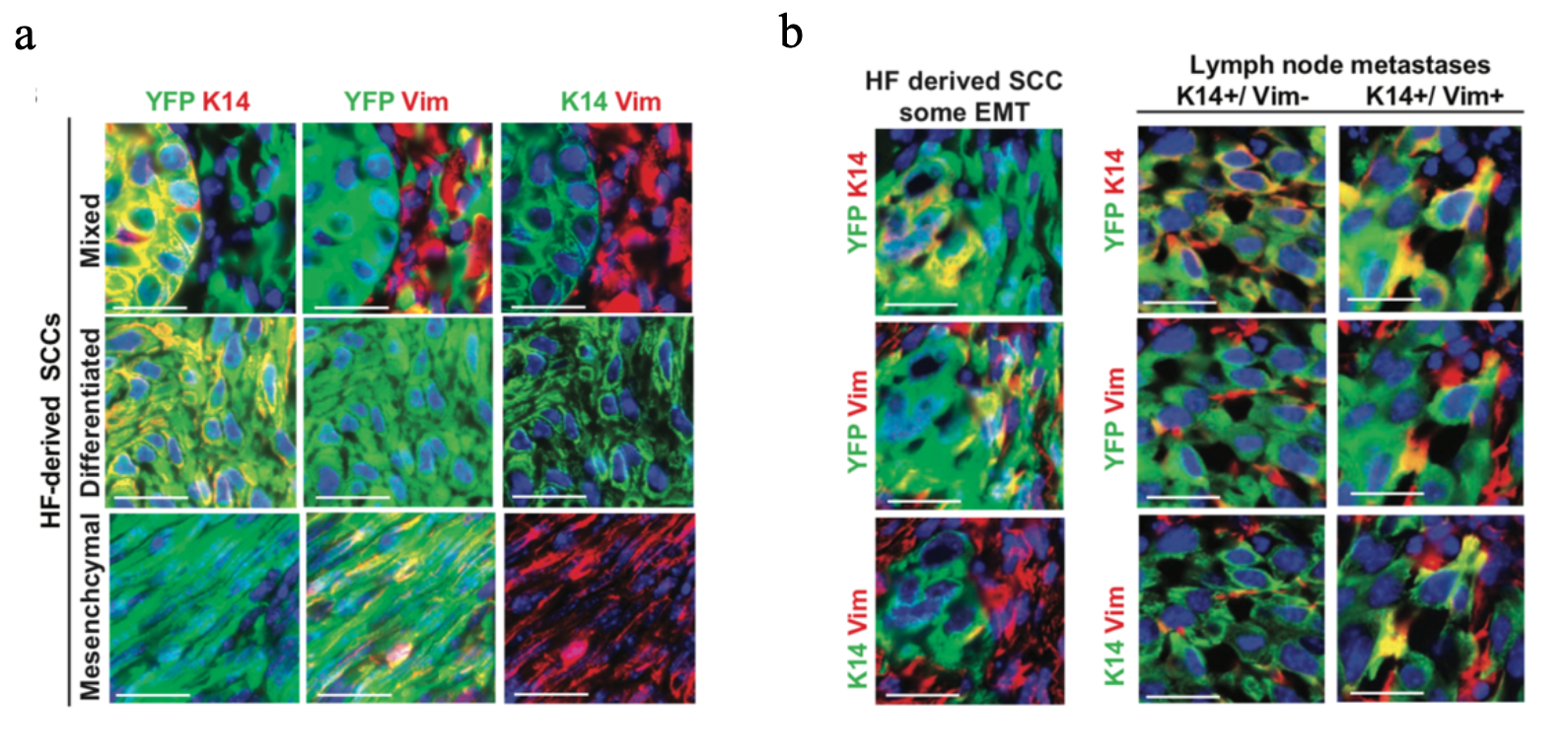
Figure 1: HF-derived SCC tumours present spontaneous EMT/MET and are associated with metastasis (Revenco et al., 2019). a Immunofluorescent images of K14 and Vimentin expression of mixed SCCs (upper panel), differentiated SCCs (middle panel), and mesenchymal SCCs (lower panel) in Lgr5CreER/Kras G12D /p53cKO/RosaYFP mice. Scale bars, 50 mm. b Representative immunofluorescence of K14 and Vimentin expression in a primary tumour with a partial EMT (left panel) arising from Lgr5CreER/KrasG12D / p53cKO/RosaYFP and their associated LN metastases (right panel). Scale bars, 50 mm (Revenco et al., 2019).
Interestingly, EMT is reversed by the mesenchymal-to-epithelial transition (MET). This process is thought to participate in metastasis formation by allowing circulating tumour cells to regain epithelial properties and extravasate into distant organs and tissues and support stabilisation and proliferation of secondary tumours (Brabletz et al., 2018). SCC tumours were used to investigate the correlation between EMT/MET and spontaneous lung and lymph node metastasis formation. The vast majority of lung metastasis arising from HF-derived SCCs models presented differentiated epithelial features (71% K14+, Vim-) with only 4% of lung metastases being purely mesenchymal (K14-, Vim+) supporting the notion that MET plays an important role for the establishment and outgrowth of metastasis from primary skin SCC (Revenco et al., 2019).
However, IFE-derived tumours did not show any signs of EMT and transplantation of tumours into immunodeficient mice always resulted in metastasis regardless of EMT. This highlights the fact that EMT activation does not occur with the same frequency between cancer types and is not a pre-requisite for tumour progression(Revenco et al., 2019).
To better understand the dynamics and molecular mechanisms that control EMT, a chromatin accessibility assay (ATAC-seq.) and transcriptional profiling in IFE- and HF-derived SCCs were performed revealing distinct chromatin and transcriptional landscapes for each specific state. Key epithelial and mesenchymal TFs were enriched and found to induce and shape EMT-specific phenotypes, elucidating the gene regulatory networks that control the gene expression program for each state (Latil et al., 2017).
Overall these studies underscore that EMT is not a binary process but rather a continuous spectrum of epithelial and mesenchymal phenotypes and – together with the reverse MET process – leads to increased metastatic potential. Each EMT transition state is associated with specific changes in morphology, gene expression, chromatin remodelling and transcription factors that define EMT as a highly dynamic process involved in cancer progression. The functional interactions occurring in this phenomenon not only reflect cancer heterogeneity but also demonstrate that more mechanistic studies are necessary to investigate the balance and regulation between the different EMT states. This will allow the progression of our understanding in tumour development and help unveil new therapeutic strategies to combat metastatic cancer. However, EMT and MET proved not to be the only factors in metastasis formation. Thus, we are still missing pieces that help us fully understand the steps between tumour initiation and metastasis. This leaves many exciting questions to explore in the future.
References and further reading
Brabletz, T., Kalluri, R., Nieto, M.A., Weinberg, R.A., 2018. EMT in cancer. Nat Rev Cancer 18, 128–134. https://doi.org/10.1038/nrc.2017.118
Gupta, P.B., Pastushenko, I., Skibinski, A., Blanpain, C., Kuperwasser, C., 2019. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 24, 65–78. https://doi.org/10.1016/j.stem.2018.11.011
Latil, M., Nassar, D., Beck, B., Boumahdi, S., Wang, L., Brisebarre, A., Dubois, C., Nkusi, E., Lenglez, S., Checinska, A., Vercauteren Drubbel, A., Devos, M., Declercq, W., Yi, R., Blanpain, C., 2017. Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell Stem Cell 20, 191-204.e5. https://doi.org/10.1016/j.stem.2016.10.018
Pastushenko, I., Blanpain, C., 2019. EMT Transition States during Tumor Progression and Metastasis. Trends in Cell Biology 29, 212–226. https://doi.org/10.1016/j.tcb.2018.12.001
Pastushenko, I., Brisebarre, A., Sifrim, A., Fioramonti, M., Revenco, T., Boumahdi, S., Van Keymeulen, A., Brown, D., Moers, V., Lemaire, S., De Clercq, S., Minguijón, E., Balsat, C., Sokolow, Y., Dubois, C., De Cock, F., Scozzaro, S., Sopena, F., Lanas, A., D’Haene, N., Salmon, I., Marine, J.-C., Voet, T., Sotiropoulou, P.A., Blanpain, C., 2018. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468. https://doi.org/10.1038/s41586-018-0040-3
Revenco, T., Nicodème, A., Pastushenko, I., Sznurkowska, M.K., Latil, M., Sotiropoulou, P.A., Dubois, C., Moers, V., Lemaire, S., de Maertelaer, V., Blanpain, C., 2019. Context Dependency of Epithelial-to-Mesenchymal Transition for Metastasis. Cell Reports 29, 1458-1468.e3. https://doi.org/10.1016/j.celrep.2019.09.081
Roche, J., 2018. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 10, 52. https://doi.org/10.3390/cancers10020052
Yeung, K.T., Yang, J., 2017. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol 11, 28–39. https://doi.org/10.1002/1878-0261.12017



