BEYOND CELL MIGRATION: NOVEL ROLES OF ROCK-MYOSIN II IN CANCER
By Lisa Backwell | Thursday 24 October 2019
In this week’s IGMM external seminar series, Professor Victoria Sanz-Moreno from Queen Mary University of London treated us to a quick-fire snapshot of her labs extensive work on an aggressive form of skin cancer called melanoma, which has led to the recent discovery that a pathway called ROCK-myosin II not only helps melanoma cells to spread but also allows them to become more invasive by co-opting the immune system.1 Melanoma is a highly dangerous form of skin cancer and the 5th most common cancer in the UK.2 Its danger lies in its ability to quickly spread (metastasise) to other areas of the body and it is this spreading which is reported as being responsible for 90% of cancer related deaths.3
Metastasising melanoma cells migrate to other areas of the body by crossing the barriers of blood vessels. They typically do this through a single-celled form of movement called rounded-amoeboid migration, however there are many methods in which cells migrate.4 The Sanz-Moreno group visualised this mode of melanoma migration by microscopy using a variety of models. Firstly, they used three-dimensional collagen matrices which mimicked the dermis (the site melanoma cells invade) and secondly, using human and mouse melanoma samples. This conclusively showed that the cancer cells which tend to spread, located at the edge of the tumour (called the invasive front) were round and highly contracted.5
In order to migrate the cell needs to contract and this is controlled by a kinase called ROCK and a protein called myosin II known to transform the cell cytoskeleton (a microscopic structure that gives cells their shape). ROCK can be stimulated by mechanical tension, or by small molecular switches called Rho GTPases.6 In contracted melanoma cells, the lab found higher levels of a phosphorylated MLC2 (a marker for high myosin II activity). Understanding the ROCK-Myosin pathway and how it leads to cell migration could therefore lead to a more specified way of preventing metastasis.
SHOULD MELANOMA PATIENTS BE TAKING ANTIOXIDANTS?
One striking observation made by the group was a correlation between low contractility and high levels of reactive oxygen species. They found on exposing poorly contracted cells to antioxidants, the cells rounded up.7 This is worrying as many cancer patients are recommended to take antioxidant supplements.8 This highlights the need for faster, better communication between the research community, the health media and the public.
MELANOMA CELLS CHANGE THEIR IMMUNE MACHINERY IN ORDER TO MIGRATE
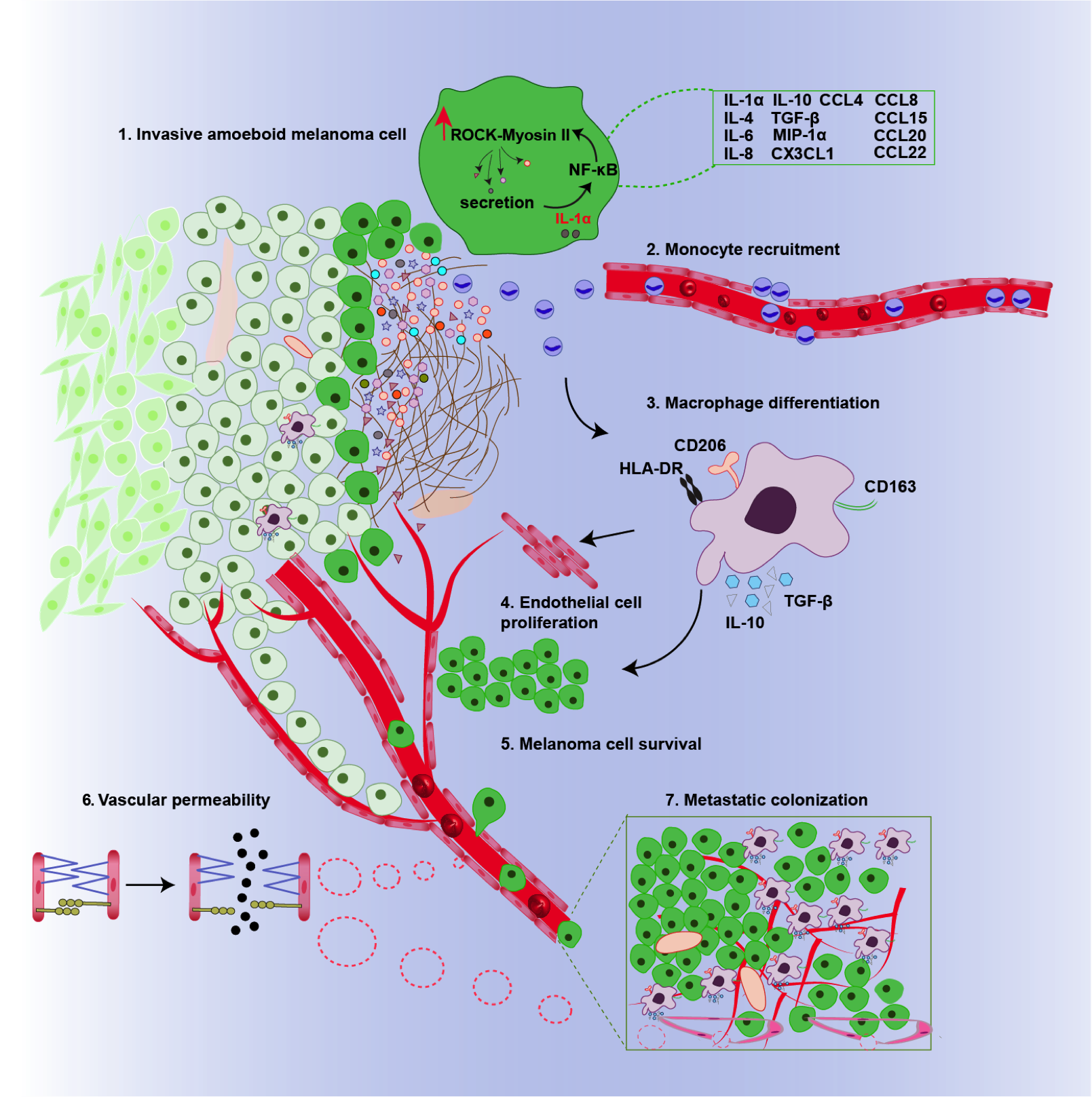
To gain some insight into what proteins, RNA molecules and metabolites are required for contractility, the group used a computational approach called OMICS to generate fingerprints of both highly metastatic more rounded melanoma cell lines and poorly metastatic melanoma cell lines. The results revealed highly metastatic cells had increased levels of proinflammatory chemicals such as IL-1α and TGF-β. They then linked these increased levels with the ROCK-myosin II pathway by knocking out ROCK and MLC2 in metastatic cells and measuring IL-1α and TGF-β secretion. Indeed, IL-1α and TGF-β secretion was reduced in the absence of ROCK and myosin II.1
Using fluorescence techniques on the two melanoma samples, Sanz-Moreno observed that the rounder more invasive cells had higher levels of immune cells called macrophages and that these were concentrated at the invasive front. Typically, macrophages help to kill cancer cells by consuming foreign bodies. However, further experiments in cells and pre- clinical models showed that IL-1α and TGF-β transform the macrophages so they support tumour growth. Moreover, IL-1α was also shown to make tiny holes in the blood vessels, facilitating cell mobility by allowing cells to easily pass the bloodstream.1
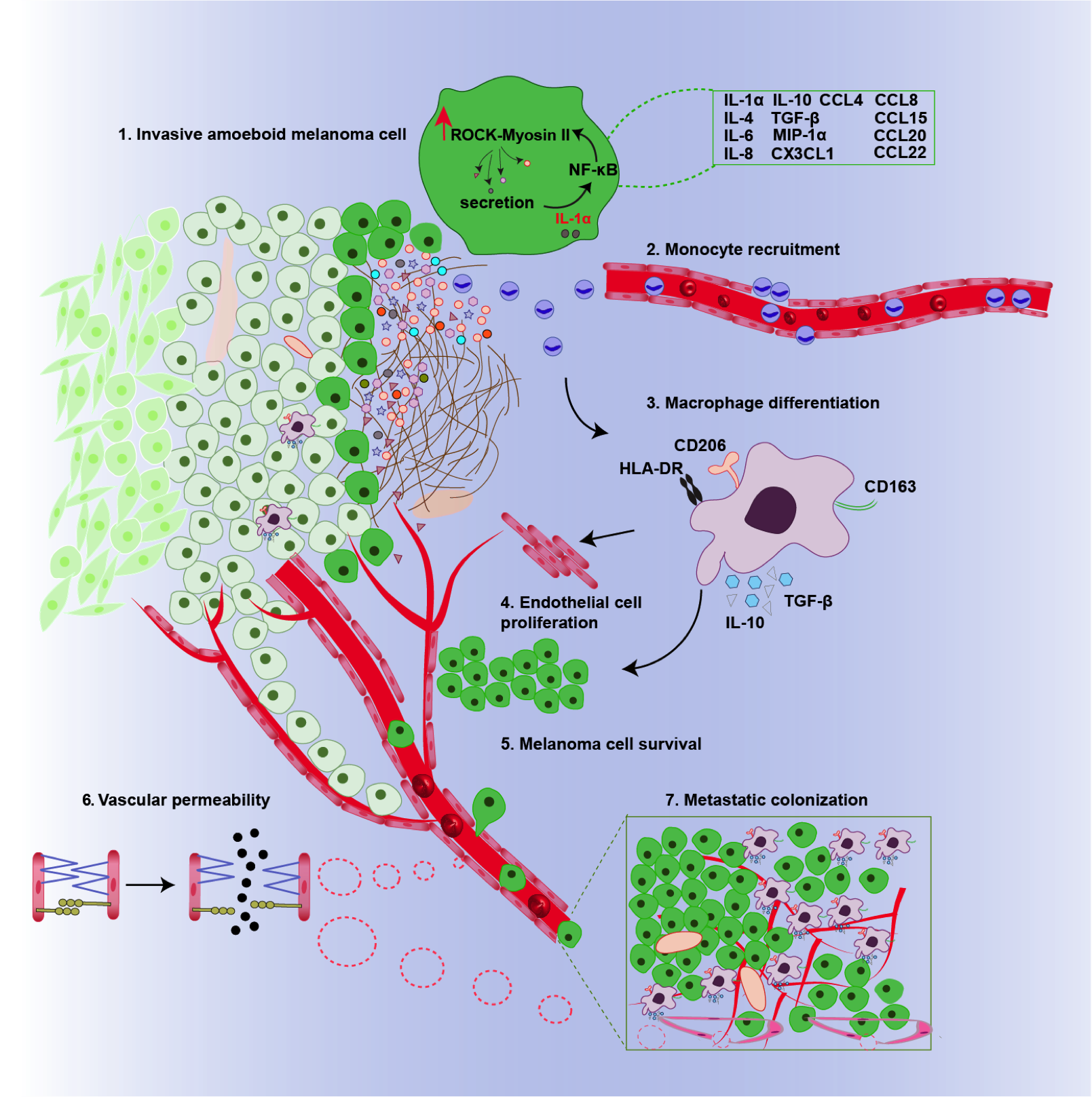
Graphical summary of the pathways influenced by the ROCK-Myosin II pathway in melanoma cells. Rounded amoeboid cells high in myosin II induces the secretion of proinflammatory chemicals such as IL-1α that induce tumour-supporting macrophages. © 2019 Prof Victoria Sanz-Moreno All Rights Reserved.1
Not only have these findings illustrated that melanoma cells can interact with their surrounding environment in order to strengthen their survival, the Sanz-Moreno lab have shown the ROCK-Myosin pathway can impact a large range of cellular mechanisms, which in turn, broadens the scope of metastasis-targeted treatment. One such treatment proposed is a topical cream of ROCK inhibitors, reactive-oxygen species, and IL-1 α –blocking antibodies that could be applied after the primary melanoma lesion is removed. This seminar showed how crucial bioinformatics is to efficiently direct research and how combining this with a variety of wet-lab techniques from microscopy to cellular biology helps to generate a more thorough analysis of complex disease mechanisms.
References and further reading
- M. Georgouli, C. Herraiz, E. Crosas-Molist, B. Fanshawe, O. Maiques, A. Perdrix, P. Pandya, I. Rodriguez-Hernandez, K. M. Ilieva, G. Cantelli, P. Karagiannis, S. Mele, H. Lam, D. H. Josephs, X. Matias-Guiu, R. M. Marti, F. O. Nestle, J. L. Orgaz, I. Malanchi, G. O. Fruhwirth, S. N. Karagiannis and V. Sanz-Moreno, Cell, 2019, 176, 757-774.e23.
- Cancer Research UK, https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer#heading-Zero, Accessed October 2019
- B. Weigelt, J. L. Peterse and L. J. van ’t Veer, Nat. Rev. Cancer, 2005, 5, 591–602.
- P. Pandya, J. L. Orgaz and V. Sanz-Moreno, Mol. Oncol., 2017, 11, 5–27.
- V. Sanz-Moreno, C. Gaggioli, M. Yeo, J. Albrengues, F. Wallberg, A. Viros, S. Hooper, R. Mitter, C. C. Féral, M. Cook, J. Larkin, R. Marais, G. Meneguzzi, E. Sahai and C. J. Marshall, Cancer Cell, 2011, 20, 229–245.
- I. Rodriguez-Hernandez, G. Cantelli, F. Bruce and V. Sanz-Moreno, F1000Research, 2016, 5.
- V. Sanz-Moreno, G. Gadea, J. Ahn, H. Paterson, P. Marra, S. Pinner, E. Sahai and C. J. Marshall, Cell, 2008, 135, 510–23.
- The Skin Cancer Foundation, https://www.skincancer.org/blog/can-your-diet-help-prevent-skin-cancer/, Accessed October 2019



