After a long Covid-19-enforced hiatus, we finally made it back to Switzerland to conduct a set of dehydration experiments on gypsum (as part of my NERC grant). The goal was to document the effects of pore fluid pressure and differential stress on the reaction. Disclaimer - we don't know in detail yet, as the results all look pretty similar. It will need a proper quantitative image analysis to figure out. What we did see though was the effect of differential stress - and lack thereof - on compaction and also the orientation of the growing hemihydrate crystals.
So, as a reminder, the reaction you see in the image below is CaSO4.2H2O => CaSO4.0,5H2O + 1,5H2O. CaSO4.0,5H2O is a mineral called hemihydrate, or bassanite. Hemihydrate grows in long needles. The reaction produces about 30% negative volume change in the solid phases (because a lot of water is released from the gypsum if heated above 60 °C). In tectonics, that water is considered critically important, as, if it cannot escape, it will increase the pore fluid pressure significantly and this render the system mechanically unstable.
In our experiments, we applied a confining pressure of 20 MPa, or 200 bars, and, to speed things up a bit (time is precious at Synchrotron light sources), we increased the temperature at the sample to 123 °C. The sample in the image below further experiences a pore fluid pressure of 50 bars and no differential stress (i.e. it is in a hydrostatic stress state, which means that the horizontal and vertical forces are equal).
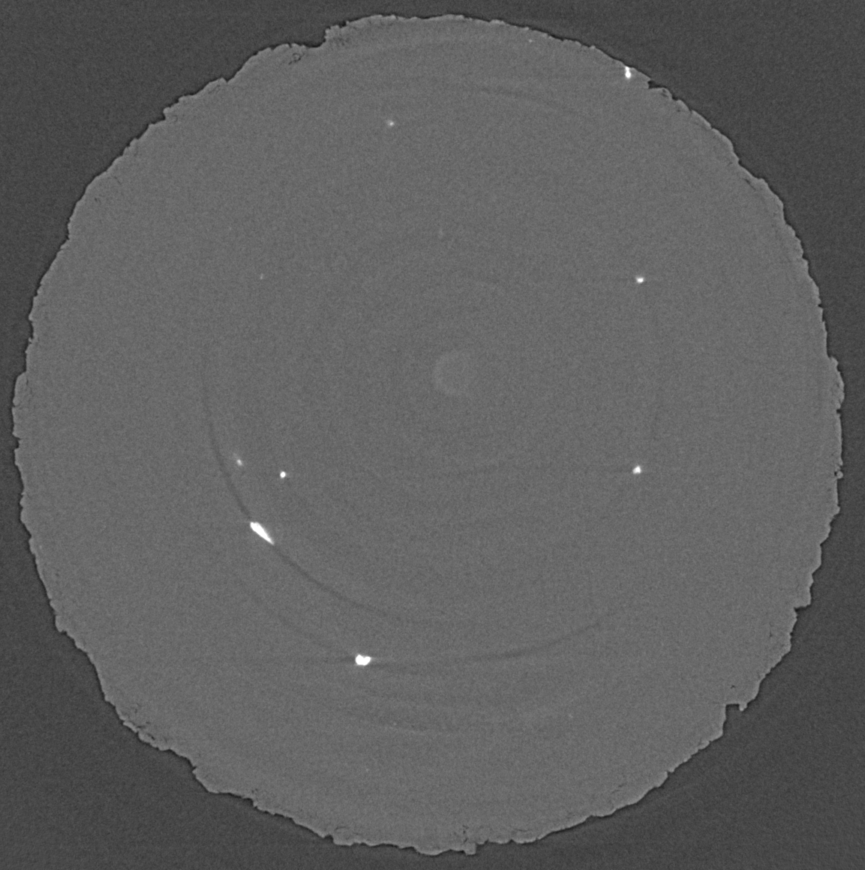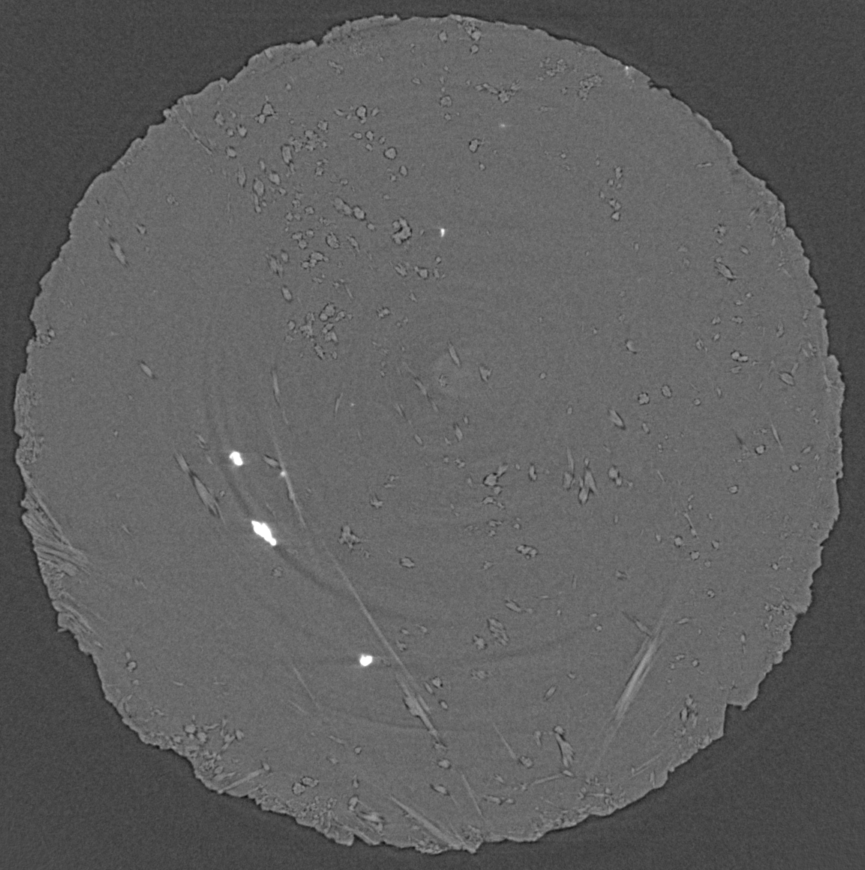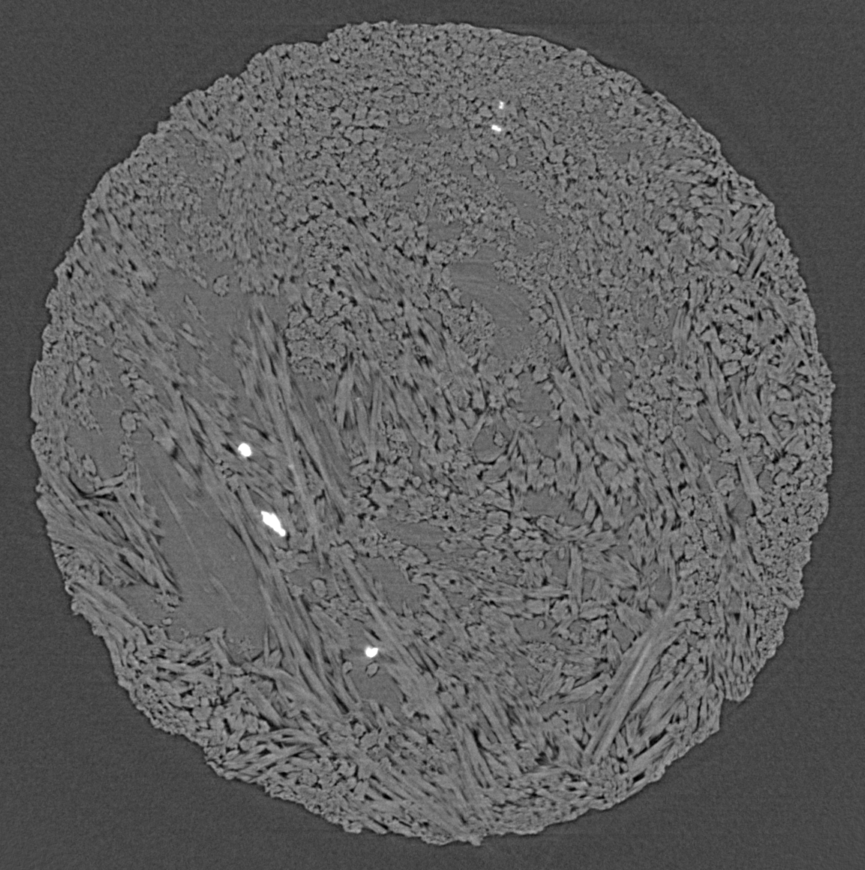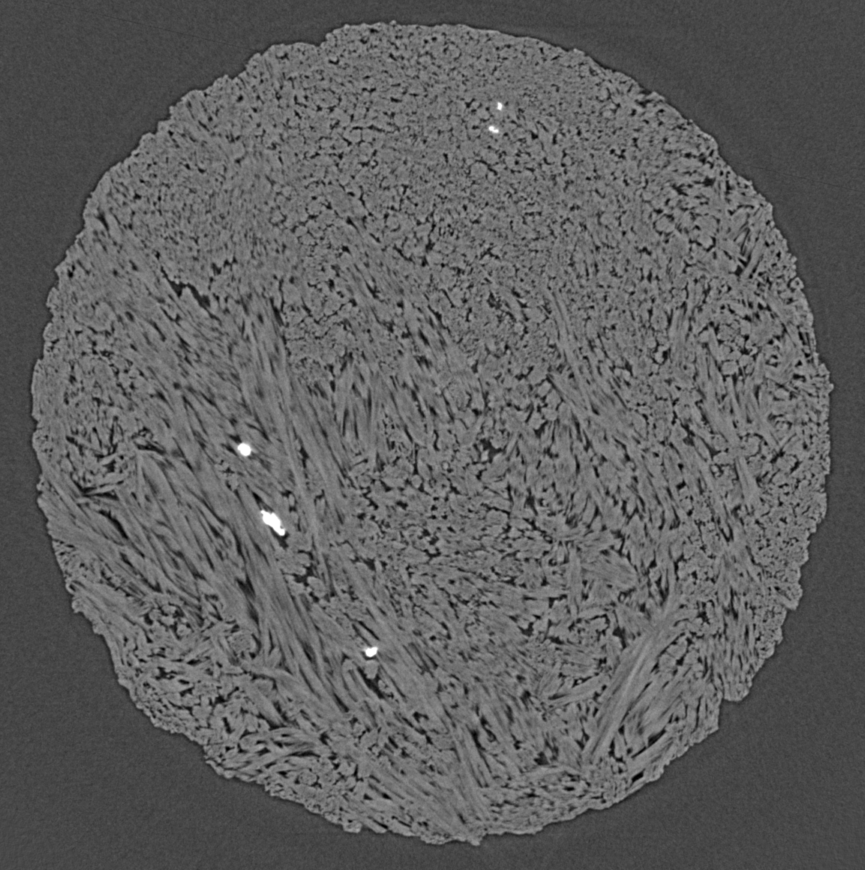
In the picture, you can see hemihydrate needles grow and replace alabaster (a gypsum rock) in different orientations, although most of them are pointing towards you (and you see them in "head cuts"). The sequence covers about an hour worth of reaction. In x-ray microtomography, we don't necessarily see grain boundaries, which is why, at the beginning of the experiment, everything appears uniformly grey. The hemihydrate needles are slightly less dense than gypsum and thus appear in a somewhat lighter grey. What is also obvious is the porosity (which stems from the negative volume change, see above), which appears as dark grey/black. A first for us to document is the shrinkage of the sample (or "compaction" in proper terms), which is cause by the confining pressure (200 bar, remember).
Once at home, we will start quantifying the changes we can see in this image. That includes proportion of gypsum/hemihydrate, from which we can derive a reaction rate. We can also follow the nucleation rate and grain growth of hemihydrate grains, their orientations and fabrics they form, but also the porosity, and how drainage evolves throughout the experiments. Lastly, we hope to be able to quantify how strain is distributed throughout the sample and over time. By doing these analyses for experiments we conducted at different conditions (pore fluid pressures, differential stresses), we hope to be able to draw a clearer picture of how dehydration reactions can influence tectonic processes such as large-scale thrusting. This will be supported by using our data to calibrate computer simulations of the process, which we do in collaboration with John Wheeler from the University of Liverpool. With the data we have acquired over the past three days, we are quite confident that we will achieve that.
Today we are helping Oliver Plümper from Utrecht University to acquire 4D data from a KBr-KCl replacement reaction (I am sure he will post on this). Tomorrow it's our turn again, and we will be testing a new experimental device we have been building over the past 10 months or so, 'Heitt Mjölnir', which is already sitting in our workbench here (see picture below), ready to go. With 'Heitt Mjölnir' we will be able to continue our dehydration experiments with gypsum but, more importantly, also with serpentinite. This is a type of rock that forms from basalts that have reacted with sea water, and forms part of oceanic crust. Serpentinite dehydration is thought responsible for the nucleation of earthquakes in subduction zones. Understanding the processes that lead from dehydration to an earthquake is a kind of holy grale in tectonics, and the ultimate goal of our research. Being able to observe, document and quantify the processes in 4D data is getting us a huge step closer achieving that.



