Dr Martin Reijns is a senior research fellow working in the Medical Research Council Human Genetics Unit. He was part of a team of university and NHS scientists who set up a COVID-19 diagnostics lab from scratch. Martin tells us how they did it.

Dr Martin Reijns in the lab
Dr Martin Reijns is one of the scientists who took on a huge challenge at the start of the COVID-19 pandemic – to transform university labs normally used for scientific research into a COVID-19 testing facility and to develop an accurate test. With NHS Lothian and his colleagues at the Medical Research Council Institute of Genetics and Molecular Medicine, they created capacity for 1,000 COVID-19 tests a day for NHS patients in Scotland.
“It all started with my boss, Professor Andrew Jackson, realising at the start of the pandemic that the countries performing the highest numbers of COVID-19 tests also had the lowest infection and death rates,” says Martin. “We wanted to help increase testing capacity for Scotland. Because a test for presence of a virus is based on techniques we normally use in our molecular biology lab, he felt that we should be able to set up COVID-19 testing fairly quickly and easily. Well, it wasn’t quick or easy, but we did do it; from scratch.”
“Everyone in the world was looking for the reagents to do COVID-19 testing so they were in short supply. At the beginning I was sitting in my living room phoning companies and sales reps and saying ‘we are setting up this facility, we will be doing lots of testing, what can you provide at what cost and how quickly?’”
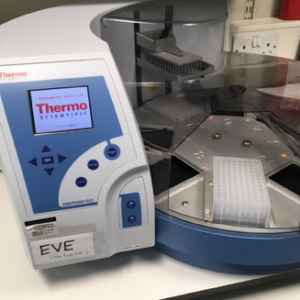
Introducing Eve, the robot
Robots
Martin and his colleagues built a COVID-19 diagnostics lab in a space usually busy with genetics researchers. Labs from all over the University of Edinburgh lent them the robots and machines they needed to create capacity for around 1,000 COVID-19 tests a day for NHS patients in Scotland. The team of volunteer scientists that set up the testing lab agreed that robots need names, they settled on characters from the film Wall-E and named them EVE, WALL-E, AUTO, BURN-E and GO-4.
Before commercial tests were available, countries all over the world shared their in-house testing protocols on the World Health Organisation website. This info helped the team build an accurate test that could be used locally, giving NHS staff and patients test results within 24 hours.
“You can do tests where you look for a single target in the virus genome- the genetic information that tells us if a person is infected – but to be more confident, it’s best to try to detect more than one target within the viral genome. So that’s what we did – our test seeks multiple targets in a single reaction. This really helps with speed and cost, and means we can be really confident in the test result,” says Martin.
The robots and machines helped speed the whole testing process up. EVE arrived in the lab first.
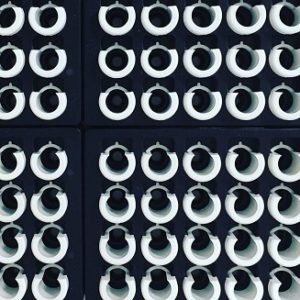
Every-day lab equipment played an essential role in testing.
In addition to the robots and machines, there were everyday lab tools at the heart of the COVID-19 test. These simple circles are tube holders, pipette tips and 96-well PCR plates. Scientists use pipettes to move precise volumes of liquid from one tube to another. The tubes and 96-well plates they move them into carry the patient sample from test start to finish. Bar codes keeps track of which patient’s test is where.
Working with samples
Martin describes what happens when the patient samples arrive: “A sample arrives as a swab in a liquid, and then a scientist from our team of volunteers carefully takes some of this liquid and inactivates the virus to make the sample safe to work with. We’ve developed a test ourselves that looks for multiple targets in the coronavirus’ genetic material, reducing the chance of false negative results. Also, we can be more confident that when there is virus in the sample, we actually get a positive test result.”
“The first step of taking it out of the swab tube has to be done by a person. It takes quite some time because it has to be done safely and of course it is crucial that the sample is linked to the correct patient and the clinician who submitted it.”
Once the sample is safe to handle and logged into an IT system, a purification step (using a robot) takes place to isolate genetic material from any virus present. Then it’s run through a procedure called RT-qPCR, which stands for reverse transcriptase quantitative polymerase chain reaction. It means making lots of copies of the genetic code the virus carries in a continuously repeating cycle.
“One big benefit of the RT-qPCR machines is that they show how much virus is present in the sample in real time; the more there is, the quicker you will see it. Another plus side is that once the sample is in the machine it needs no further handling; the machine does everything else. It sends results to a computer programme that then allows a scientist to see if there is virus in the patient sample,” says Martin.
The testing team was staffed by volunteers from the University of Edinburgh – a call went out at the start of lockdown and more than 1,000 scientists responded immediately.
“There were a lot of people who wanted to help, but it was decided to invite people already familiar with our building, some of whom even had previous experience of handling infectious disease samples. We now have a team of about 25 scientists supported by our local NHS colleagues who’ve been working throughout the pandemic to run COVID-19 tests for the NHS,” says Martin.
“We have capacity to run 1,000 tests a day, but so far the most we’ve needed to do in one day was around 300. In the vast majority of cases, we have results back with hospital staff within 24 hours.”

Researcher at Medical Research Council Unit The Gambia.
Sharing test protocols
The team developed an accurate test that could show whether there was actually virus in patient samples. This test can be used alongside similar tests that have since become available commercially. They have also shared the test protocol and even reagents with their colleagues at another Medical Research Council funded lab in The Gambia, Africa, and are hoping to share the methodology more widely by publishing it.
Martin says: “The first thing we did was send our colleagues in The Gambia enough reagents for 5,000 COVID-19 tests, as they were struggling to get hold of the reagents they needed. As demand is still increasing for them, they asked if we can provide kit for another 20,000 tests. We can help them without having any impact on our own testing capability so I’m trying to organise that now. About 2.5 million people live in The Gambia, so it’s about half the size of Scotland. With our help they can further expand their COVID-19 testing capabilities, by creating capacity at the MRC Unit The Gambia. It’s quite gratifying to know that the test we are sharing with them is going to help people in a whole other country.”
“Although it was hard under the circumstances, developing this test was absolutely the right thing to do, so I’d do it again. I’m happy that I was able to contribute.”



2 Comments
2 Pingbacks