Working together to unveil the genetics behind Rare Disease – collaborative PhD projects
How can early career researchers, such as PhD students, integrate the expertise of several labs into their research? This post aims to introduce why we research rare diseases, particularly craniofacial disorders, and share the experiences of students working across labs with complementary expertise.
Congenital malformations are conditions which emerge early in development. Researching the genetics linked to these rare congenital diseases can help us understand fundamental principles and mechanisms more broadly applicable to development, health, and other diseases.
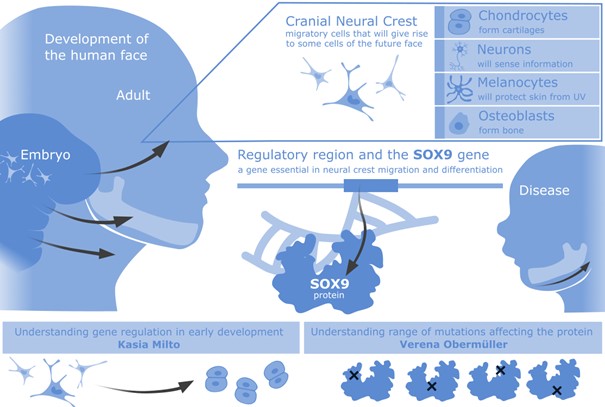
The development of the face involves the emergence of neural crest cells, which will migrate long distances to form key parts of the face. The neural crest cells will give rise to a network of neurons. Additionally, uniquely in the head, it will give rise to a range of other cell types, including chondrocytes and bone progenitors, which will form cartilages and other facial structures (Figure 1).
The Long lab is interested in the complex regulation of a particular gene involved in congenital diseases affecting the face – SOX9. This gene is essential in the neural crest maturation to become facial structures (1). Both the errors in the SOX9 protein structure and its regulation can cause disease. Errors in SOX9 protein are involved in Campomelic Dysplasia (CD) (2), where the ability of the protein to execute its role is affected and leads to a complex developmental disorder (affecting sex specification, limbs and the face). Interestingly, subtle changes in other parts of the genome can affect the dose and tissue specificity of SOX9 expression, which can also cause diseases such as Pierre Robin Syndrome (PRS) (3). In PRS, the coordination of expression of SOX9 is disrupted only during jaw development (4), leading to a smaller jaw and cleft palate, while other tissues are unaffected. The exact mechanisms behind CD and PRS are yet to be fully elucidated.
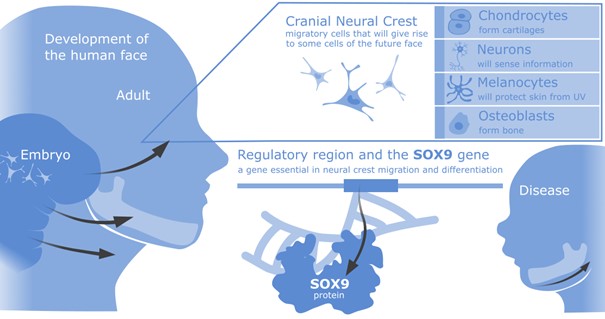
Figure 1. Face in health and disease – the SOX9 gene and the neural crest population that arise in early development to migrate and give rise to a range of facial structures found in the cranial (head and neck) region.
To get a fuller picture, researchers across several groups collaborate across the Institute of Cancer and Genetics. What does the everyday work of PhD students who explore the mechanisms involved in SOX9-related rare diseases look like? I have the privilege of sharing the insights and experiences of two young scientists:
Kasia Milto, an ECAT clinical lecturer and PhD candidate interested in understanding SOX9 role in the earliest events in development, leading onto CD. I have asked her about her experience coming from the clinic and working in collaboration between two labs, combining expertise from gene regulation lab (Long lab) and early development lab (Nichols lab).

Could you tell us about your experience working in a collaboration between two labs?
“I consider myself lucky to be working with two very inspirational supervisors, Prof Jenny Nichols and Dr Hannah Long. Jenny’s group studies the early stages of mammalian development, from formation of the blastocyst to implantation, gastrulation and the onset of organogenesis. Hannah’s group investigates mechanisms of gene regulation in the context of craniofacial development and how perturbations of SOX9 gene expression can lead to human congenital craniofacial disorders, such as campomelic dysplasia.
“My project brings those interests together to model campomelic dysplasia and understand mechanisms of SOX9 gene regulation in neural crest specification and function, by employing early stem cell-based models of human development.
“It is not always easy to juggle multiple lab meetings, journal clubs and seminars (not to mention social events! 😊) but being part of two research groups only enriches my learning. I am developing a diverse skillset and unique understanding of the topic I study, while receiving support from both supervisors and multiple lab members. I particularly enjoy how my supervisors bring different perspectives to the project and novel ways of tackling biological questions.’’
As a Medic, can you tell us more about potential collaborations you envision in future?
‘’Plastic and Reconstructive Surgery is an incredibly varied and collaborative speciality. We work on all areas of the body and with patients of all ages. In the context of paediatric plastic surgery, we often work closely with paediatric doctors, clinical geneticists, ear nose and throat surgeons, speech therapists, anaesthetists, clinical psychologists, hospital play therapists, specialist nurses and many more. In the NHS such multidisciplinary teams bring their expertise together for the best shared decision-making for our patients.
“Both my supervisors regularly work with the NHS. Jenny collaborates with assisted conception clinics that share early human embryos, generally deemed unsuitable for embryo transfer, kindly donated to her research projects with informed consent from anonymous couples undergoing treatment. Hannah works closely with clinical geneticists who see patients with craniofacial malformations and has presented her research at the UK Clinical Excellence Network (CEN) for cleft genetics.
“I can certainly envision myself contributing to developing closer links between basic research and the clinic. This could be especially valuable for patients and families of children with rare diseases, for whom evidence-based medicine in the form of clinical trials might be lacking. I am still conceptualising the collaborative project I could develop, but I hope that having access to potential tissue samples and clinical phenotype information could be of value. The first step will be raising awareness of excellent scientific advances made at the MRC HGU among plastic surgeons in Scotland and brainstorming ideas on how we could contribute as clinicians. I believe that we are steadily moving towards personalised medicine in the future and close links between clinics and research groups could only benefit our patients.’’
Verena Obermüller is a PhD student working under co-supervision from labs with expertise in synthetic biology (Kudla), computational analysis of disease-causing mutations (Marsh ) and genome regulation (Long). Verena’s research aims are to gain a quantitative understanding of the mutations found in CD and research their impact on the function of the SOX9 protein. I have asked her about her experience working on a rare disease in a collaborative project she has organised.
“I think the best thing about having more than one supervisor is that you get to know many people with expertise in different areas, so whenever I need help with something there is usually someone around I can ask. That’s not just limited to people from the labs of my supervisors, but from other groups as well. Since there are many collaborative projects going on at the Institute, it seems like the different groups are quite well connected. The same way this is benefitting me, I also hope that I can introduce people from my different groups to each other and help move their projects forward.
“It can be really helpful to get feedback from different experts and hear different perspectives, especially when I am at a point where I need to decide what direction I want to move the project in.”
In summary, we hope sharing these experiences will encourage new collaborations across research groups and fields, and further forge strong connections between researchers to address complex research challenges from multiple angles.
References
- Spokony RF, Aoki Y, Saint-Germain N, Magner-Fink E, Saint-Jeannet JP. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development. 2002 Jan 15;129(2):421–32.
- Unger S, Scherer G, Superti-Furga A. Campomelic Dysplasia. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2024 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1760/
- Robin P. A fall of the base of the tongue considered as a new cause of nasopharyngeal respiratory impairment: Pierre Robin sequence, a translation. 1923. Plast Reconstr Surg. 1994 May;93(6):1301–3.
- Long HK, Osterwalder M, Welsh IC, Hansen K, Davies JOJ, Liu YE, et al. Loss of Extreme Long-Range Enhancers in Human Neural Crest Drives a Craniofacial Disorder. Cell Stem Cell. 2020 Nov 5;27(5):765-783.e14.



