From two to many: centriole production in multiciliated cell differentiation
I have always regarded centrosomes as the organelles responsible for organising microtubules, with the primary function of nucleating microtubules during mitosis. However, after attending a seminar by Alice Meunier from L’Institut de Biologie de l’ENS Paris, I can certainly say that the world of centrosomes is a lot more complex.
In addition to its well-known role in microtubule production for mitosis in cycling cells, centrosomes are key in the production of cilia in multiciliated cells (MCC). MCCs have 30 – 300 cilia that beat in a wave-like pattern, crucial in processes including circulation of the cerebral spinal fluid in the brain, oocyte transport and removal of toxins in the respiratory tract (Spassky and Meunier, 2017). Unsurprisingly, MCC dysfunction is implicated in diseases such as primary ciliary dyskinesia (PCD), reduced generation of multiple motile cilia (RGMC), and can cause developmental brain defects and subfertility.
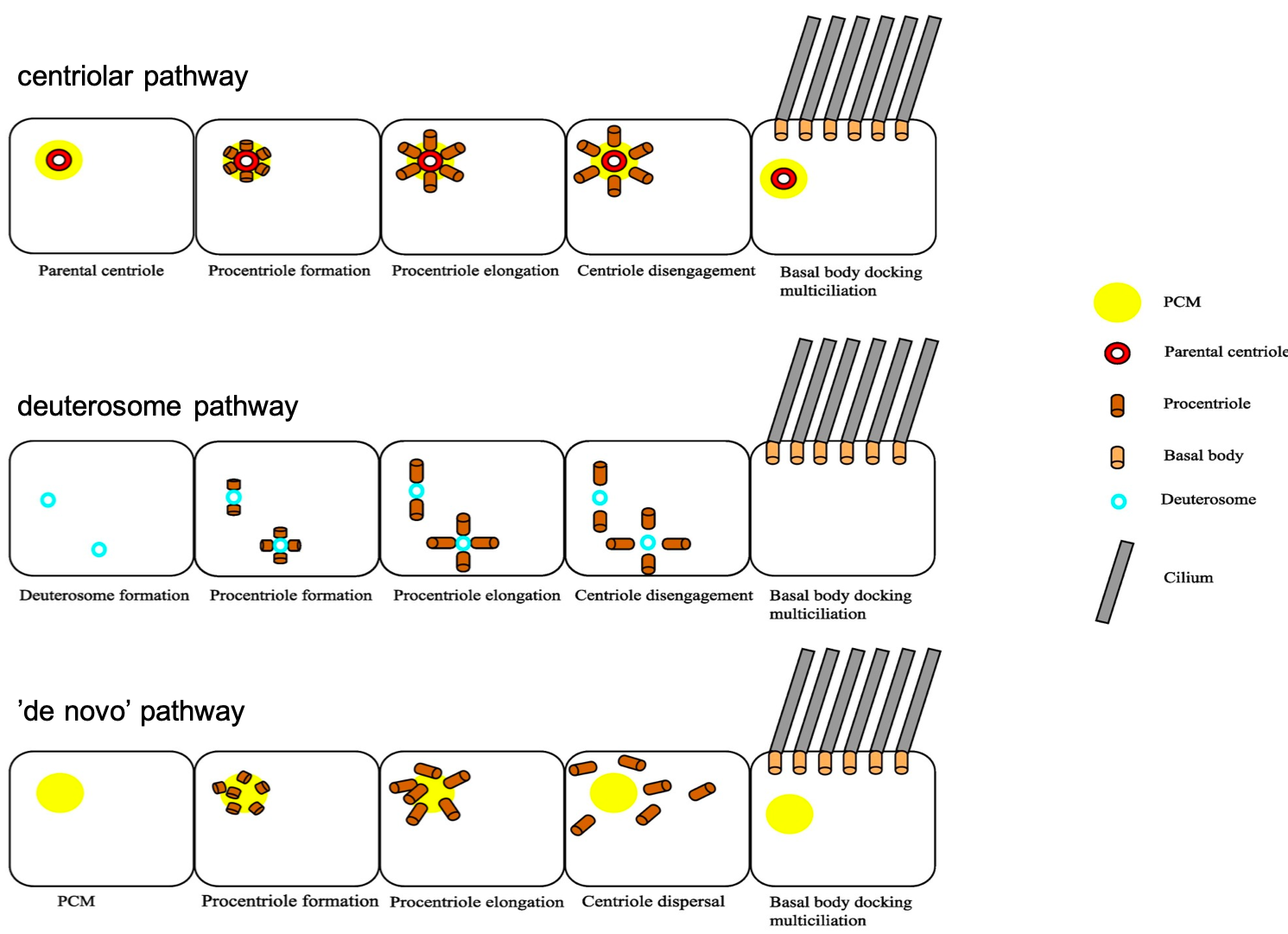
Each centrosome contains two centrioles, which must be duplicated prior to cell division. Centriole duplication occurs in parallel to DNA replication and centriole numbers must be tightly regulated to prevent genome instability and aneuploidy. To ensure accurate centriole number in cycling cells, duplication is controlled in three different ways – spatially to initiate formation in proximity to the mother centriole, temporally with only one round of duplication per cell cycle and numerically so only one daughter centriole is formed (Song et al., 2008). Interestingly, MCCs contain a unique structure termed the ‘deuterosome’, which allows them to overcome this regulation to direct mass centriole production instead. Deuterosomes are generated from the proximal side of the daughter centriole, and act as an intermediate ‘shuttle’ for procentrioles to bud off from (Al Jord et al., 2014). It is this switch from direct nucleation of procentrioles at the wall of the daughter centriole to the production of procentrioles from deuterosomes that amplifies centrioles, producing about 90% of these cilia precursors for MCC differentiation (Mercey, Levine, et al., 2019).
Work by the Meunier lab compared the deuterosome pathway for massive centriole amplification to the centriolar pathway used to duplicate centrioles. Centriole amplification occurs in a step-wise manner with three phases – amplification, growth and disengagement. As the process of centriole duplication corresponds with mitosis in a phase-dependent manner, it was possible that centriole amplification followed a similar mode of regulation. This was explored by manipulating levels of two core mitotic regulators – Cdk1 and APC/C (Al Jord et al., 2017). Firstly, when Cdk1 activity was inhibited, amplification of centrioles was prolonged, generating a greater number of procentrioles. There was also a delay in the growth to disengagement transition, resulting in incomplete centriole disengagement or complete arrest in centriole dynamics. The opposite relationship was observed when Cdk1 activity was overactivated, with accelerated centriole formation instead. When APC/C activity was blocked, centriole amplification was unaffected, but the procentrioles took a longer time to transition between the growth and disengagement phase, with some cells entering mitosis. Overall, the regulation of centriole amplification was deemed to be controlled via these mitotic regulators, with Cdk1 mediating amplification and APC/C mediating growth. This work illustrates how terminal differentiation is triggered through exploiting the same molecular players in mitosis, with APC/C activity dampening Cdk1 activity and inhibiting the mitotic machinery, thus driving centriole amplification dynamics rather than proliferation.
The second part of Alice’s talk discussed the importance of the two centriole biogenesis platforms – the parental wall and the deuterosomes. As deuterosomes develop from the surface of the daughter parental centriole, it was a logical hypothesis that removal of the parental centriole would impact procentriole production dynamics. A core centriole protein was labelled with GFP and procentriole amplification was assessed by live imaging (Mercey, Al Jord, et al., 2019). When differentiation was triggered in the absence of a parental centriole, deuterosomes were still able to arise without an impact on final procentriole numbers or dynamics. Similarly, when deuterosomes were depleted from cells, procentriole amplification also occurred with usual dynamics. So, it seemed that there was redundancy between both methods of procentriole production. But if both deuterosomes and parental centrioles were depleted, surely procentriole amplification rates would be altered? Once again, this paper proved this to be untrue, as procentrioles were somehow still able to form from clouds of pericentriolar material via a ‘de novo’ pathway. These results have shown that centriole biogenesis platforms are dispensable and highlighted the levels of robustness in cilia production. However, with greater understanding of this topic also comes an increasing number of questions. How do procentrioles arise from pericentriolar material and what are the cellular components required for this? If deuterosomes are dispensable and centrioles can form normally without them in MCC differentiation, why did they evolve in the first place? Whilst these results have been surprising and contradictory to previous ideas on deuterosome and parent centriole importance, the Meunier lab hopes to lead the way in a search for answers regarding the puzzle of centriole biogenesis.
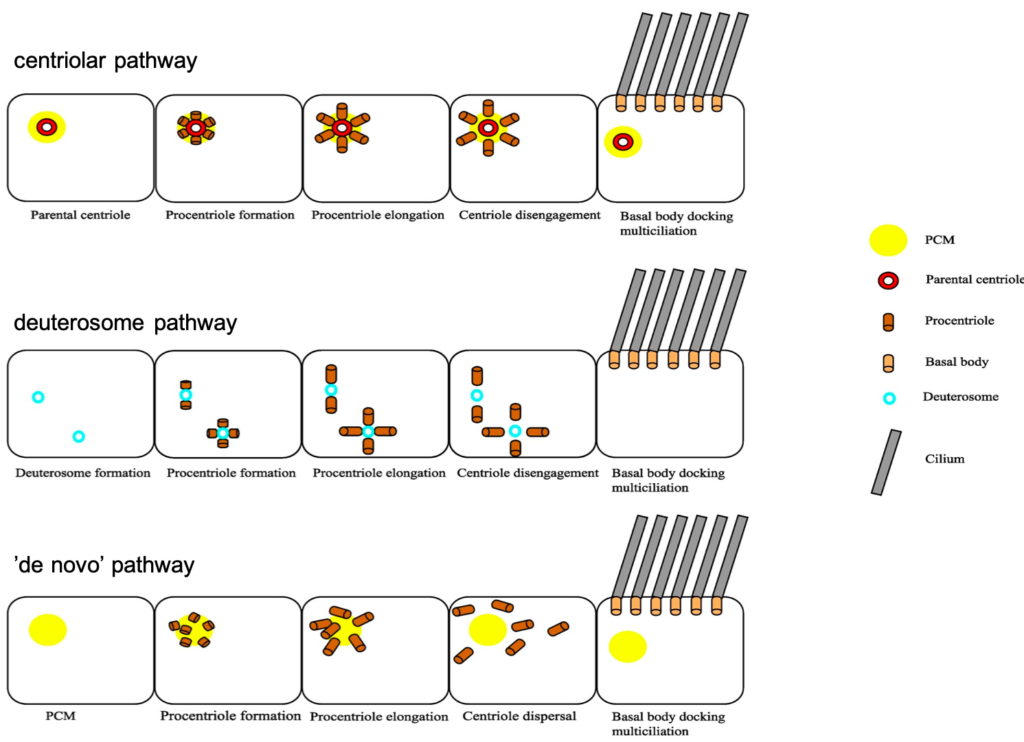
Image modified from Rayamajhi and Roy, 2020.
References and Further reading:
Al Jord, A. et al. (2014) ‘Centriole amplification by mother and daughter centrioles differs in multiciliated cells’, Nature. Nature Publishing Group, 516(7529), pp. 104–107. doi: 10.1038/nature13770.
Al Jord, A. et al. (2017) ‘Calibrated mitotic oscillator drives motile ciliogenesis’, Science. American Association for the Advancement of Science, 358(6364), pp. 803–806. doi: 10.1126/science.aan8311.
Mercey, O., Al Jord, A., et al. (2019) ‘Dynamics of centriole amplification in centrosome-depleted brain multiciliated progenitors’, Scientific Reports. Nature Publishing Group, 9(1), pp. 1–11. doi: 10.1038/s41598-019-49416-2.
Mercey, O., Levine, M. S., et al. (2019) ‘Massive centriole production can occur in the absence of deuterosomes in multiciliated cells’, Nature Cell Biology. Nature Research, 21(12), pp. 1544–1552. doi: 10.1038/s41556-019-0427-x.
Rayamajhi, D. and Roy, S. (2020) ‘Multiciliated Cells: Rise and Fall of the Deuterosomes’, Trends in Cell Biology. Elsevier, 0(0). doi: 10.1016/j.tcb.2020.02.003.
Song, M. H. et al. (2008) ‘Centrioles: some self-assembly required’, Current Opinion in Cell Biology. NIH Public Access, pp. 688–693. doi: 10.1016/j.ceb.2008.09.001.
Spassky, N. and Meunier, A. (2017) ‘The development and functions of multiciliated epithelia’, Nature Reviews Molecular Cell Biology. Nature Publishing Group, pp. 423–436. doi: 10.1038/nrm.2017.21.



