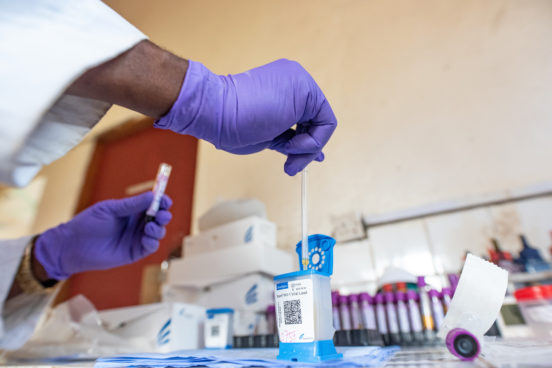
As Covid-19 pandemic expands its global reach, increasing testing capacity has taken centre stage in government and international agendas. Drawing on research and policy engagement in Sierra Leone, the DiaDev (Investigating Diagnostic Devices in Global Health) team at the University of Edinburgh show the critical importance of investing in laboratory capacity. New diagnostic devices are only effective insofar as they can be integrated into the broader health system and supported by continuous supply chains, trained medical staff and closely aligned information systems.
“We have a simple message for all countries” declared Dr Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization. “Test, test, test.” Accurate diagnosis is essential to mitigate the increasingly disastrous impact of the COVID-19 outbreak. Without knowing who among the general population is sick or has previously been infected, policy makers are flying blind, facing unpredictable surges in cases, health workforce shortages and an interminable cycle of lockdowns and forced closures.
But while the economic and public health rationale of mass testing is irrefutable, if the past two months of this pandemic has taught us anything, it is that following Tedros’ mandate is hardly straightforward. Rapidly developing tests from scratch and deploying them widely demands clinical, commercial and regulatory coordination and, above all, a sufficiently-prepared and well-integrated laboratory system.
As the outbreak moves into the African continent, the question of diagnostic capacity looms large. A position piece, published last week in the African Journal of Laboratory Medicine, offers a key perspective on what is needed for robust diagnostic response in an outbreak and the role tests can play in building resilient health systems. Co-authored by the DiaDev team at the University of Edinburgh and Kings College London and policy-makers, doctors, public health experts, laboratory scientists from Sierra Leone, the paper reflects on efforts to scale up diagnosis during the Ebola outbreak, the longer-term impact of those investments on the health system and provides some key lessons for the COVID-19 response in Africa and more widely.
Diagnostic tests need diagnostic systems
At the root of the 2014–2016 Ebola Outbreak was an inability to quickly diagnose and isolate cases. With unprecedented speed, a range of novel Ebola diagnostic tools, from automated PCR machines designed for laboratory benchtops to rapid test kits that could be used at the point of care, were trialled in Sierra Leone, helping to bring the outbreak to an end. But while important, increasing the availability of tests was only a first step. To safely transport samples, source reagents, dispose of hazardous materials, and correctly interpret and feed-back diagnostic data into clinical and public health decision-making necessitated health system-wide support.
The extent to which laboratory strengthening efforts during the Ebola outbreak have prepared West African countries for Covid-19 remains uncertain. One important legacy in Sierra Leone is a national cohort of laboratory workers with experience of PCR testing. A number of GeneXpert PCR machines, which can be repurposed for SARS-COV-2 testing, also remain in country. But prioritisation during the outbreak of disease-specific Ebola tests, to the detriment of broader laboratory strengthening efforts, means weak supply chains and waste management systems remain major points of vulnerability across the region.
National experts and institutions need to be fully engaged
When it comes to the ready deployment of global health innovations in Africa, regulatory capacity is often neglected. In an effort to accelerate R&D for Ebola diagnostics, the World Health Organization developed the WHO Emergency Use Listing (EUL) to expedite the evaluation of new tools in the epidemic. While ostensibly the aim was to alleviate the regulatory burden on National Regulatory Agencies (NRAs), without local input or support, national agencies struggled to register the influx of new tests. Regulatory authorities can be advocates for new medical products, but need manpower and expertise to evaluate device performance, guide deployment and procurement and to provide the quality assurance and post-market surveillance essential for safeguarding patients and health staff. The leadership of the Africa CDC in coordinating diagnostic capacity in response to the Covid-19 outbreak has meant national experts are more likely to be heard. A modified EUL procedure launched for COVID-19 places increased emphasis on the role of NRAs, but for regulatory alignment to be feasible this must be accompanied by enhanced resources, training and investment.
Africa’s Diagnostic Futures
Africa is the next frontier for the pandemic. At the time of writing, the number of confirmed cases is near 10,000. What epidemiological realities lie behind that number is unclear, as diagnostic capacities across the continent differ widely.
Currently, there are more than 100 rapid point-of-care devices for Covid-19 in the pipeline, and the global health organisation FIND is assisting African governments with evaluating rapid tests for Covid-19 coming onto the market. But the emphasis on novel tests, while important, distracts from interventions that are just as critical for a successful response while building capacity for the future.
The Sierra Leonean experience makes clear that investment in new tests is just the starting point. If COVID-19 is going to be contained, substantial investments must be made in national laboratory networks and the supply chains, waste management systems, and health information infrastructures that support them. This is the key to building strong laboratory systems for the next epidemic.
An earlier version of this piece was published on 9th April 2020 on the Kings College London Covid-19 website.
Images included in this essay feature laboratory workers and cleaners working at health facilities in and around Freetown and were taken by Olivia Acland for the DiaDev research project.
Research for this article was undertaken as part of the ‘Investigating Diagnostic Devices in Global Health’ research project (www.diadev.eu) and was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme grant agreement No 715450.













Comments by