The needs of public health and the economy need to be finely balanced during the pandemic, write Farah Huzair and Joyce Tait
Our work on previous emerging infectious diseases has built up a wealth of knowledge that we are bringing to bear on Covid-19. This paper brings together some of this research: (i) demonstrating the importance of understanding human behavioural dynamics, (ii) supporting the role of innovation in diagnostics, drugs and vaccines for emerging infectious diseases, and (iii) justifying more rapid, adaptive regulatory systems, as part of an enabling innovation ecosystem.
In the run-up to the declaration of a Covid-19 pandemic, there have been major reactions in financial markets, with global recession and longer term structural adjustment on the cards. However, previous emerging infectious diseases have built up a wealth of knowledge that we are bringing to bear on Covid-19. Pandemic preparedness was initiated during the SARS outbreak in 2002. SARS was not declared a pandemic, but health care advisors became quickly attuned to the threat of emerging zoonotic diseases. SARS was followed by the H5N1 event in 2005, the H1N1 pandemic in 2009 (resulting in an estimated 84,000 deaths worldwide (1), MERS in 2012, H7N9 in 2013 and Ebola in 2019, all of which were thought to have pandemic potential at the time of outbreak. Throughout these previous challenges, as with Covid-19, governments have been faced with the mutually incompatible challenges of encouraging social distancing to minimise the spread of the disease and encouraging healthy people to go to work as usual to minimise the impact on the economy.
Each event has added to the body of knowledge that might be used by governments, regulators and health agencies on how to manage pandemic events and improve the chances of a quick recovery.
Understanding human behaviour
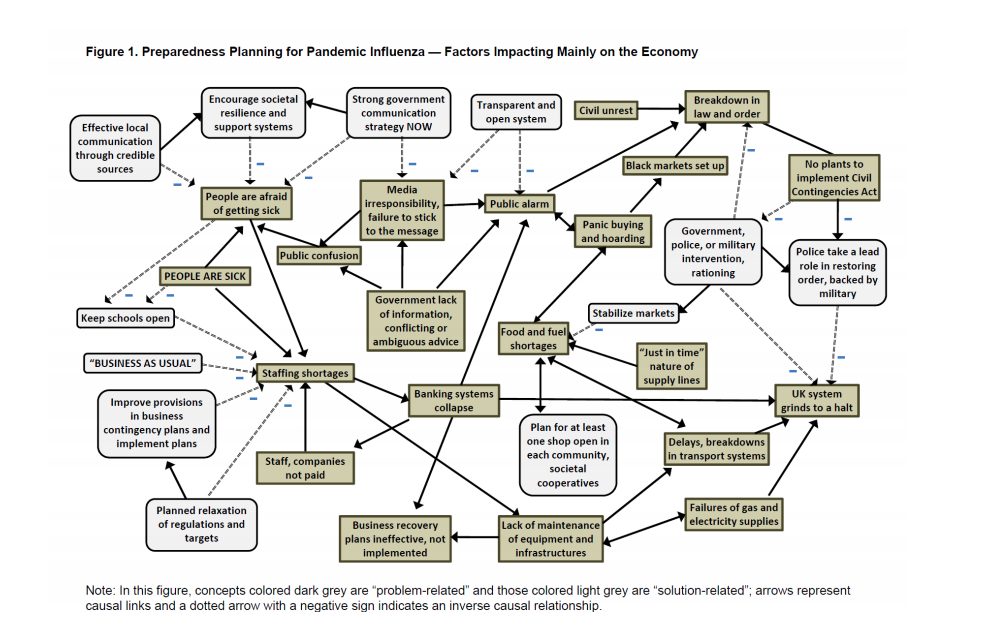
The H5N1 epidemic did not, as had been feared, evolve to enable human-to-human transmission and the infection from birds to humans was restricted largely to East Asian countries. However, the case fatality rate (CFR) was ~60% leading to alarm and serious contingency planning for a pandemic outbreak. In the UK at the time, pandemic preparation included the prospect of simultaneously giving contradictory public messages – “business as usual” and “social distancing”, potentially leading to serious disruption of the economy (2) (Figure 1). The research underlying tis figure analysed the views of emergency responders on the UK’s preparedness plans, probably the best informed group to make such comments. It also looked at the economic value of a vaccine, given the expected tendency towards “prophylactic absenteeism” (top left-hand side of Figure 1), where healthy people would avoid going to work in case of contracting infection (3). Modelling the impact of the disease on UK GDP showed that, although prophylactic absenteeism would reduce the infection rate by ~1%, its impact on the economy would be in the billions of pounds. We proposed that the benefits of an effective vaccine or drug should be calculated, not just on the basis of its health impacts, but also on its economic value in giving people the confidence to continue to go to work. This study highlighted the importance of the expected CFR in determining the extent to which prophylactic absenteeism would occur. In the H1N1 event, the ‘problem-related’ behaviours described in Figure 1 began to appear in several countries, including the UK, but rapidly evaporated when it became clear that the CFR was similar to normal winter flu. This eliminated the need for the vaccines and drugs that had been made rapidly available in response to a higher expected CFR.
The role of innovation in diagnostics, drugs and vaccines
At the time of the H1N1 outbreak, vaccine manufacturing was dominated by a handful of multi-national corporations, with hope and trust mainly invested in standard inactivated or attenuated virus vaccines. The innovation ecosystem is now much more vibrant and varied, occupied by small, medium and large biotechnology firms, working in partnerships, consortia, and other collaborative arrangements. New scientific discoveries in synthetic biology, gene editing, and other biotechnologies are enabling small, agile and dynamic firms to develop radically new approaches to diagnosis and, potentially, treatment for Covid-19 (4). For example, Geovax (US) and BravoVax (Wuhan, China) are developing a vaccine using a ‘plug-and-display’ technology platform that uses virus-like particles and genetic material specific to Covid-19. This approach has been used to produce vaccines for Zika, Lassa fever and Ebola. iBio (US) and CC-Pharming (China) are developing a vaccine in plants that combines automated hydroponics, vertical farming systems and plant bioreactor technology to rapidly scale-up production. This has already been used to produce antibody candidates for Ebola, Dengue fever, HPV, seasonal and avian influenza. LineaRx (US) and Takis Biotech (Italy) have produced a synthetic gene to be delivered to muscles for the temporary generation of an antigen which could trigger an immune response against Covid-19 (5). APEIRON Biologics AG in Austria has a recombinant human enzyme product (APN01) which is already approved for other indications (e.g. acute lung injury) and is being trialled in Covid-19 patients in China in partnership with Angalpharma Co., Ltd (China) and dMed Pharmaceutical Co. (China) (6). Impressively this work is being undertaken without the coordination activities of the WHO which, during H1N1, developed and circulated both the seed strain and reagent and facilitated data sharing between vaccine producers (7).
More rapid, adaptive regulatory systems
During the H1N1 pandemic, the European Medicines Agency undertook significant regulatory adaptation, with new expedited review and licensing procedures (8). In April 2009, the new strain was identified and characterised. On June 11th, the WHO declared a pandemic, allowing fast track assessment of mock-up vaccines and rolling review of vaccine quality. Non-clinical and clinical pharmacovigilance (RMP) data and labelling information were submitted to the regulator by the Marketing Authorisation Holders. The timeframe for evaluation of the vaccines was reduced from 210 days to 70 days (9).
The H5N1 and H1N1 events also stimulated new approaches to health communication between public health agencies, healthcare organizations and frontline clinicians (11) and review and updating of risk assessment and management procedures (12).
Lessons for Covid-19
Covid-19 is again demonstrating the difficulty of adopting the necessary social distancing to protect the health of the population without also creating severe economic repercussions. Most governments have prioritised ‘social distancing’ over protecting the economy (‘business as usual’), even though the CFR for the majority of people seems to be low enough to avoid public panic. At the time of writing it is not clear how necessary this action is or how effective it will be. However, Covid-19 has re-emphasised the over-riding importance of setting up a new globally coordinated research programme to find more rapid ways: (i) to develop targeted diagnostics, drugs and vaccines, (ii) to scale up their production to meet the needs of global populations, and (iii) to develop routine, smarter and faster approaches to their regulation. The cost to the global economy of Covid-19 justifies whatever cost will be involved to deliver this outcome so that when the next pandemic comes along we are better prepared to deal with it.
This paper was originally published in the INNOGEN Policy Briefs: https://www.innogen.ac.uk/reports-and-commentaries
Dr Farah Huzair is the current programme director for the MSc in Management of Bioeconomy, Innovation and Governance in the department of Science, Technology and Innovation Studies, University of Edinburgh. Professor Joyce Tait is the director of INNOGEN in the department of Science, Technology and Innovation Studies, University of Edinburgh.
References
- CIDRAP (2020) “CDC estimate of global H1N1 pandemic deaths: 284,000” Available at: http://www.cidrap.umn.edu/news-perspective/2012/06/cdc-estimate-global-h1n1-pandemic-deaths-284000 Last accessed 16th March 2020.
- Tait, J. (2011) “Innovation, Policy, and Public Interactions in the Management of Infectious Diseases” Available at: http://scienceforglobalpolicy.org/wp-content/uploads/5522b96206a09-Tait.pdf. Last accessed 16th March 2020.
- Smith et al. (2009) “The economy-wide impact of pandemic influenza on the UK: A computable general equilibrium modelling experiment” BMJ;339: b4571 doi:10.1136/bmj.b4571
- Clinical Trials Arena (17th February 2020) “Covid-19: Pharmaceutical companies and agencies that partnered for coronavirus vaccine development”. Available at: https://www.clinicaltrialsarena.com/analysis/covid-19-pharmaceutical-company-partnerships-for-coronavirus-vaccines-development/. Last accessed 16th March 2020.
- Pharmaceutical technology (10th February 2020) “Applied DNA and Takis Biotech partner on coronavirus vaccine”. Available at: https://www.pharmaceutical-technology.com/news/applied-dna-coronavirus-vaccine/ . Last accessed 16th March 2020.
- Pipeline Review.com (26th February, 2020) “APEIRON’s respiratory drug product to start pilot clinical trial to treat coronavirus disease COVID-19 in China”. Available at: https://pipelinereview.com/index.php/2020022673884/Proteins-and-Peptides/APEIRONs-respiratory-drug-product-to-start-pilot-clinical-trial-to-treat-coronavirus-disease-COVID-19-in-China.html . Last accessed 16th March 2020.
- Huzair, F (2012). The influenza vaccine innovation system and lessons for PDPs. Human Vaccines & Immunotherapeutics. Vol 8, Issue 3. Available at: https://doi.org/10.4161/hv.18701. Last accessed 10 March 2020.
- Shivji, R and Purves, J. (2009) “European Medicines Agency: Influenza Pandemic Preparedness”. In Risk Wise: Epidemics. J.Griffiths and R.Lambert eds. Available at: http://digital.tudor-rose.co.uk/risk-wise-epidemics/files/assets/common/downloads/publication.pdf. Last accessed 16th March 2020.
- EMA (24th September 2009) “Pandemic influenza A(H1N1)v vaccines authorised via the core dossier procedure: Explanatory note on scientific considerations regarding the licensing of pandemic A(H1N1)v-vaccines” Available at: https://www.ema.europa.eu/en/documents/medicine-qa/explanatory-note-scientific-considerations-regarding-licensing-pandemic-ah1n1v-vaccines_en.pdf. Last accessed 16th March 2020
- EMA ‘Vaccines for Pandemic Influenza’. Available at: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/pandemic-influenza/vaccines-pandemic-influenza. Last accessed 19th March.
- Abraham, T. (2011) “Lessons from the pandemic: the need for new tools for risk and outbreak communication” Emerging Health Threats Journal, Vol 4, Issue 1. Available at: https://www.tandfonline.com/doi/full/10.3402/ehtj.v4i0.7160. Last accessed 2020.
- WHO (May, 2017) “Pandemic Influenza Risk Management”. Available at: https://www.who.int/influenza/preparedness/pandemic/PIRM_update_052017.pdf. Last accessed 16th March 2020.